Teriflunomide (Aubagio) given green light in Pakistan

MS fraternity welcomes the news that Aubagio (Teriflunomide) has been made available in Pakistan. The treatment is now available under the Named Patient Program, from October 2014.
Aubagio 14 mg is a once-daily, oral therapy indicated in the European Union and also approved by FDA for the treatment of adult patients with relapsing-remitting multiple sclerosis.
“This is an exciting development for the MS community in Pakistan who face daily challenges from their debilitating disease. The availability of Aubagio, as the first oral, once-daily, first-line medicine for the treatment of relapsing-remitting MS, offers an alternative to repeated injections which for many patients can be a real burden on top of their symptoms,” commented by one of the MS patient from Karachi.
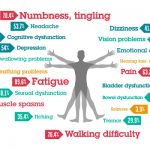
RRMS accounts for approximately eighty-five per cent of all initial diagnoses in MS. The majority of people with RRMS experience approximately one to two relapses per year. Around half of all relapses may leave people with lingering problems and disability may accumulate over time.
Aubagio’s availability in Pakistan marks an important step in improving the standard of care available to people with MS.