NOTE: This is a guest post by our sponsor, Genzyme, A Sanofi Company. They are fully funding and sponsoring the MS Awareness Seminar being organized by the Society for Multiple Sclerosis Patients in Pakistan, scheduled to take place on Saturday 30 May, 2015 at Koh-e-Noor Hall, PC Hotel Lahore, from 4:00 pm – 6:00 pm EVENT DETAILS
FDA has approved Genzyme’s AUBAGIO® (teriflunomide), a Once-Daily, Oral Treatment for Relapsing Multiple Sclerosis.
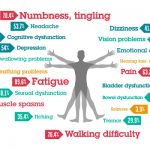
U.S. Food and Drug Administration (FDA) has approved AUBAGIO® (teriflunomide) as a new once-daily, oral treatment indicated for patients with relapsing forms of multiple sclerosis (MS). AUBAGIO has shown significant efficacy across key measures of MS disease activity, including reducing relapses, slowing the progression of physical disability, and reducing the number of brain lesions as detected by MRI.
AUBAGIO as a new treatment option can make a difference in the lives of people with multiple sclerosis.
The FDA approval was based on efficacy data from the TEMSO (TEriflunomide Multiple Sclerosis Oral) trial. In the Phase III TEMSO trial, AUBAGIO 14 mg significantly reduced the annualized relapse rate (p=0.0005) and the time to disability progression (p=0.0279) at two years versus placebo in patients with relapsing forms of multiple sclerosis. AUBAGIO 7 mg significantly reduced the annualized relapse rate (p=0.0002) in the trial.
Many people living with MS struggle with the additional burden of injectable therapies administered daily to weekly. The FDA’s approval of AUBAGIO, a new oral treatment option, is an encouraging advancement for the MS community and may be a valuable treatment for people living with this often debilitating disease.
The AUBAGIO clinical development program, involving more than 5,000 patients in 36 countries, is amongst the largest of any MS therapy. Some patients in extension trials have been treated for up to 10 years.
In MS clinical studies with AUBAGIO, the incidence of serious adverse events were similar among AUBAGIO and placebo-treated patients. The most common adverse events associated with AUBAGIO in MS patients included increased ALT levels, alopecia, diarrhea, influenza, nausea and paresthesia.
The AUBAGIO clinical development program in MS also included the recently reported TOWER study. TOWER assessed the efficacy and safety of once-daily, oral AUBAGIO in patients with relapsing forms of multiple sclerosis (MS). In the study, patients receiving teriflunomide 14 mg had a statistically significant reduction in annualized relapse rate and risk of disability progression. In addition, a significant reduction in annualized relapse rate was observed in patients treated with teriflunomide 7 mg compared to placebo. Adverse events observed in the trial were consistent with previous clinical trials with teriflunomide in MS.
AUBAGIO is an immunomodulator with anti-inflammatory properties. Although the exact mechanism of action for AUBAGIO is not fully understood, it may involve a reduction in the number of activated lymphocytes in the central nervous system (CNS).